Health Cloud® healthcare data warehouse is a database solution enabling healthcare companies to pool, reuse and store their personal health data over a long period of time for research purposes.
An offer to make full use of your health data in the public interest
CNIL-approved database for long-term storage of Personal Health Data* for analysis and research purposes in the public interest.
The Health Data Warehouse enables analyses to be carried out on a large database, with a view to improving patient care and accelerating research.
The Health Data Warehouse solution offered by Cloud Santé® is based on 4 service layers:
1. Regulatory support minimizing steps to take;
2. A secure, compliant technical infrastructure (HDH, GRPD, ISO 27001, ISO 27701, EDS referential, security referential applicable to the SNDS) ;
3. A set of tools and services to harness the wealth of data;
4. An organizational environment that ensures compliance during studies;
*SNDS: National Health Data System
General information
The French CNIL defines Healthcare Data Warehouses as databases intended for use in healthcare research, studies or evaluations.
An Health Data Warehouse makes it possible to converge all administrative and medical data from the various software programs used by healthcare professionals into a single database.
This data can then be analyzed to optimize care or for research purposes. In any event, each study carried out as part of an Health Data Warehouse must have a public-interest mission.

Health Data Warehouse will enable us to multiply the uses of massive healthcare data. It represents an opportunity to :
- Pool health data collected in different contexts, for large-scale studies;
- To store health data over a long period (10 to 20 years), for a variety of studies in the public interest;
- Reuse collected data for a purpose other than that specified at the time of collection;
- Determine the feasibility of a study by checking whether the number of patients corresponding to the inclusion criteria is sufficient;
- Set up cohorts (a population of subjects who meet a given definition and who are followed over time);
- Conduct retrospective studies;
- Identify patients.
In order to simplify the procedures for those responsible for these sensitive databases, while providing a rigorous legal and technical framework, on November 17, 2021 the French DPA published a set of guidelines relating to the processing of personal data implemented for the purpose of creating data warehouses in the healthcare field.
The aim of these guidelines is to provide a framework and facilitate the authorization process for the creation of data warehouses.
Health Data Warehouse PLAYERS
The data controller
Every Health Data Warehouse is associated with a Data Controller (Responsable de Traitement), who must implement all appropriate measures (technical and organizational) to guarantee the protection of the personal data processed. This person is also responsible for determining the purposes and means of processing.
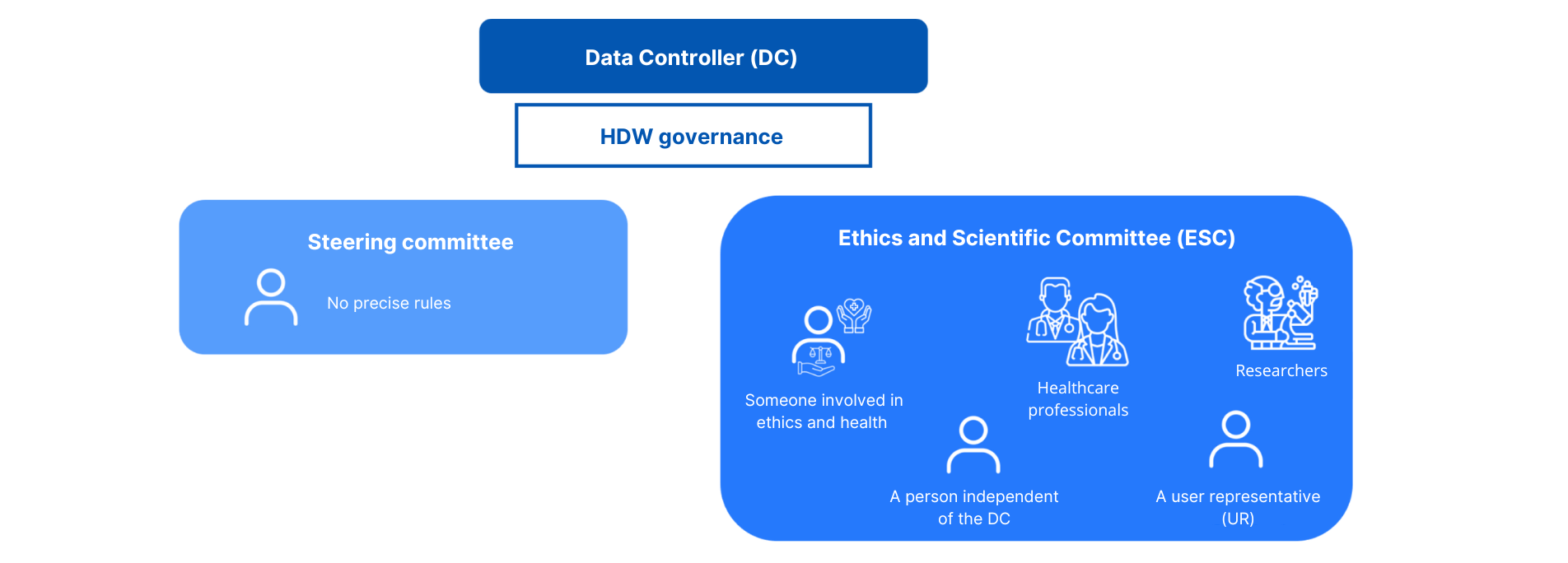
The governance
The data controller implements a governance structure for each warehouse it sets up.
This governance consists of :
- A steering committee
Role: “Determines the strategic and scientific orientations of the warehouse. Keeps an exhaustive list of the data in the warehouse, and justifies its necessity. Defined by the CNIL.
The steering committee works upstream to ensure that the warehouse can best meet the needs of researchers and caregivers.
Composition : No precise rules
- An ethical and scientific committee
Role: “Provides a prior, reasoned opinion on project proposals requiring the re-use of data from the warehouse”. Defined by the CNIL* guidelines
The Scientific and Ethical Committee examines research projects and their data analysis protocols. It issues a positive or negative opinion, which determines whether or not access to data in the warehouse is authorized, based on ethical and scientific criteria.
Composition: At least the following must be present
- A person involved in health ethics
- A person independent of the data controller
- Health and medico-social professionals
- Researchers
- A user representative (RU) or from a patient association
The patients
The data contained in an Health Data Warehouse belongs to the patient. Patients are informed of the integration of their data into the Health Data Warehouse via a personal information note.
They must also be given access to the list of studies in which their data are used. This information can be provided via the data controller’s website.
They can withdraw their consent at any time by contacting the doctor who collected the data, or directly the Health Data Warehouse Data Protection Officer (DPO).
EURIS CLOUD SANTE® HEALTH DATA WAREHOUSE CLOUD, A TOOL TO ACCELERATE INNOVATION IN HEALTHCARE.
Discover our flexible turnkey solution, designed for rapid deployment.
Customizable to your needs, Health Data Warehouse Cloud Santé® gives you the opportunity to shape your project in your own image, while guaranteeing security and regulatory compliance.
Euris Cloud Santé® supports you in setting up your Health Data Warehouse with an offer based on 4 layers of services:
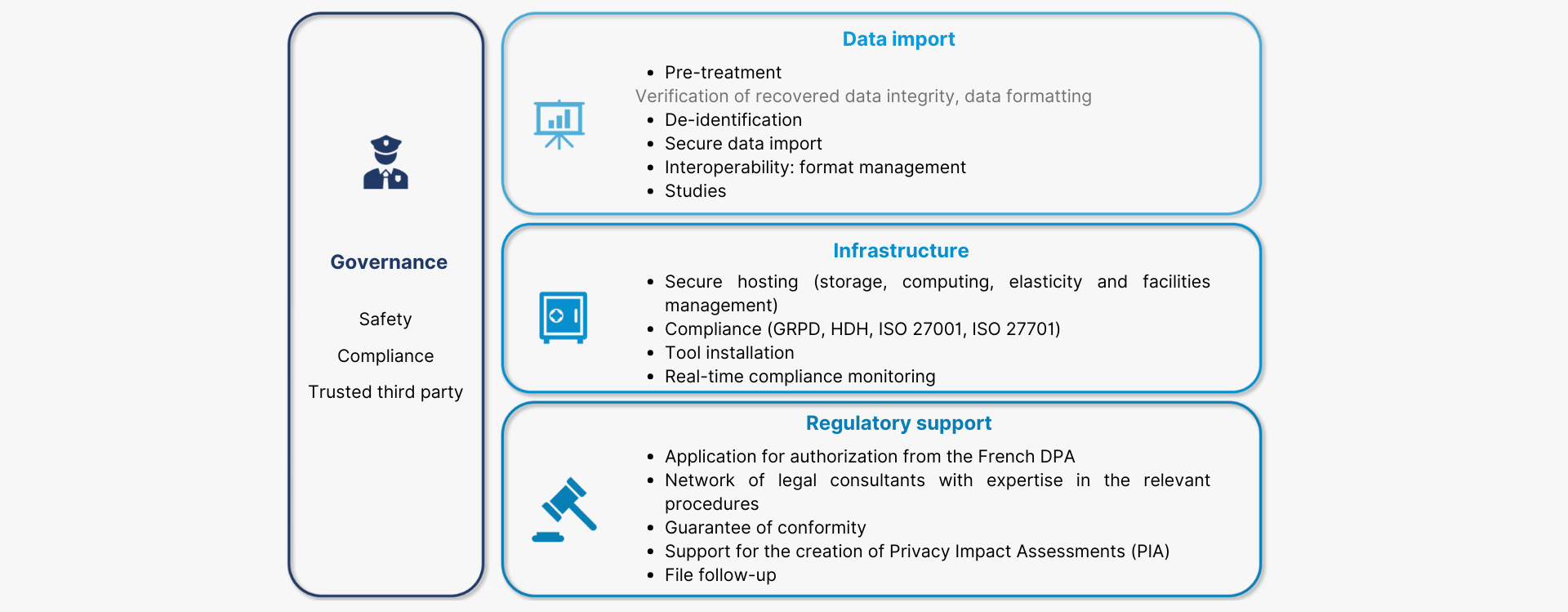
Our Health Data Warehouse consists of a central area called the “master Health Data Warehouse” and workspaces dedicated to your studies.
These workspaces are watertight, allowing you to work in parallel on different studies within the Health Data Warehouse. They can be customized according to the needs of your data scientists, and include all the tools required to carry out the study.
Our data storage infrastructure is monitored in real time to ensure consistency and security.
BENEFIT FROM A CATALOG OF PARTNERS TO DEVELOP YOUR PROJECTS THANKS TO
Tools and solutions
Data catalogs
Advice and support
ENRICH YOUR PROJECTS WITH
Business Intelligence (BI)
Analyze and use your data effectively, minimizing cognitive biases, amplifying discoveries and reinforcing data culture.
Data analysis tools
Des données synthétiques
AI-generated data facilitates access to healthcare data while guaranteeing optimal quality.
Experts in data analysis
Benefit from the expertise of healthcare professionals to analyze your data.
Federated Learning
Train collaborative machine learning models without sharing your data.
Legal support
Specialized experts in health and innovation.

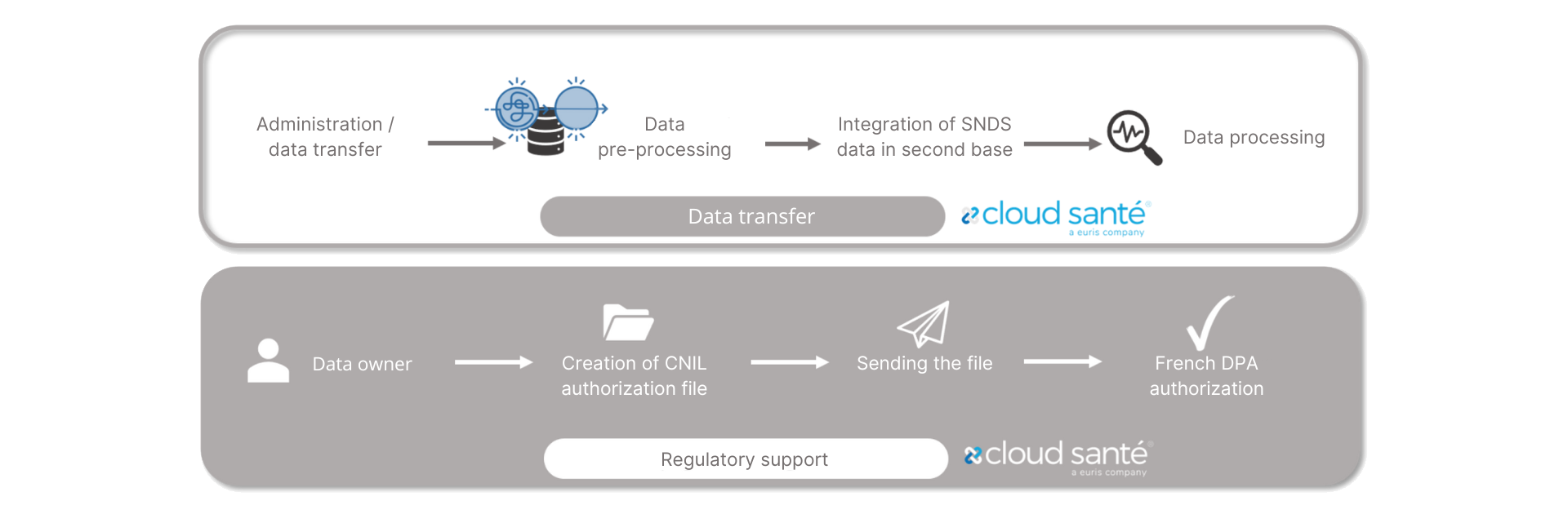
SNDS ACCES AND CHAINING
ENRICH YOUR HEALTH DATA WAREHOUSE WITH ADDITIONAL DATA FROM THE SNDS DATABASE
Thanks to its TTP accreditation, Euris transfers SNDS data securely to your Health Data Warehouse.
*TTP : Trusted third party


In its capacity as RFI*, Euris Cloud Santé® supports you in the procedures for accessing the SNDS, making these data available in your Health Data Warehouse, and using them independently and in compliance with regulations.
* RFI (=RMO): Responsible for implementation
Health expertise
Benefit from a team of experts in health IT project for more than 20 years.
E-HEALTH CERTIFICATIONS
Data security & global compliance : EU (HDS & ISO 27001), US (HIPAA), China (CSL & PHIMM).
INTERNATIONAL
Projects deployed around the world: Europe, Asia, America, Africa.
CONTINUITY OF SERVICE
High availability architecture, 99.9% SLA, 24/7 outsourcing.
PROTECTION OF YOUR PRIVATE DATA
Availability, Integrity, Confidentiality, and Auditability of Health Data.
RICH FUNCTIONALITIES
An infrastructure-as-a-Service providing cost-effective, flexible and functional solutions.

