SANA: a secure platform for multicentric studies and collaborative health data analysis
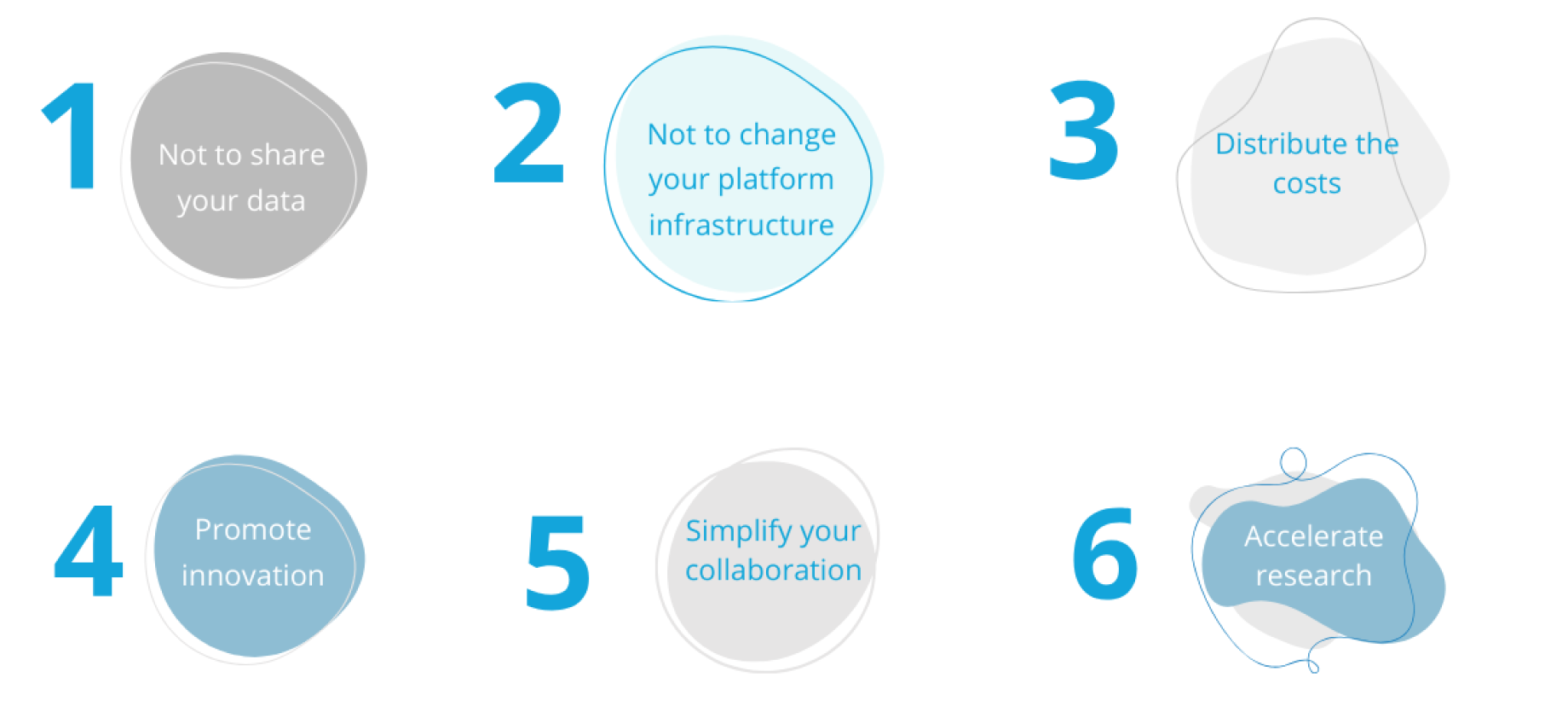
SANA offers a secure regulatory and legal framework to promote collaboration between different stakeholders or institutions.
It is now possible to collaborate with other stakeholders in the context of a multicentric study, without having to share your data or modify the infrastructure of your Health Data Warehouse (EDS). This concept ensures the confidentiality of your data while preserving your original technical environment.
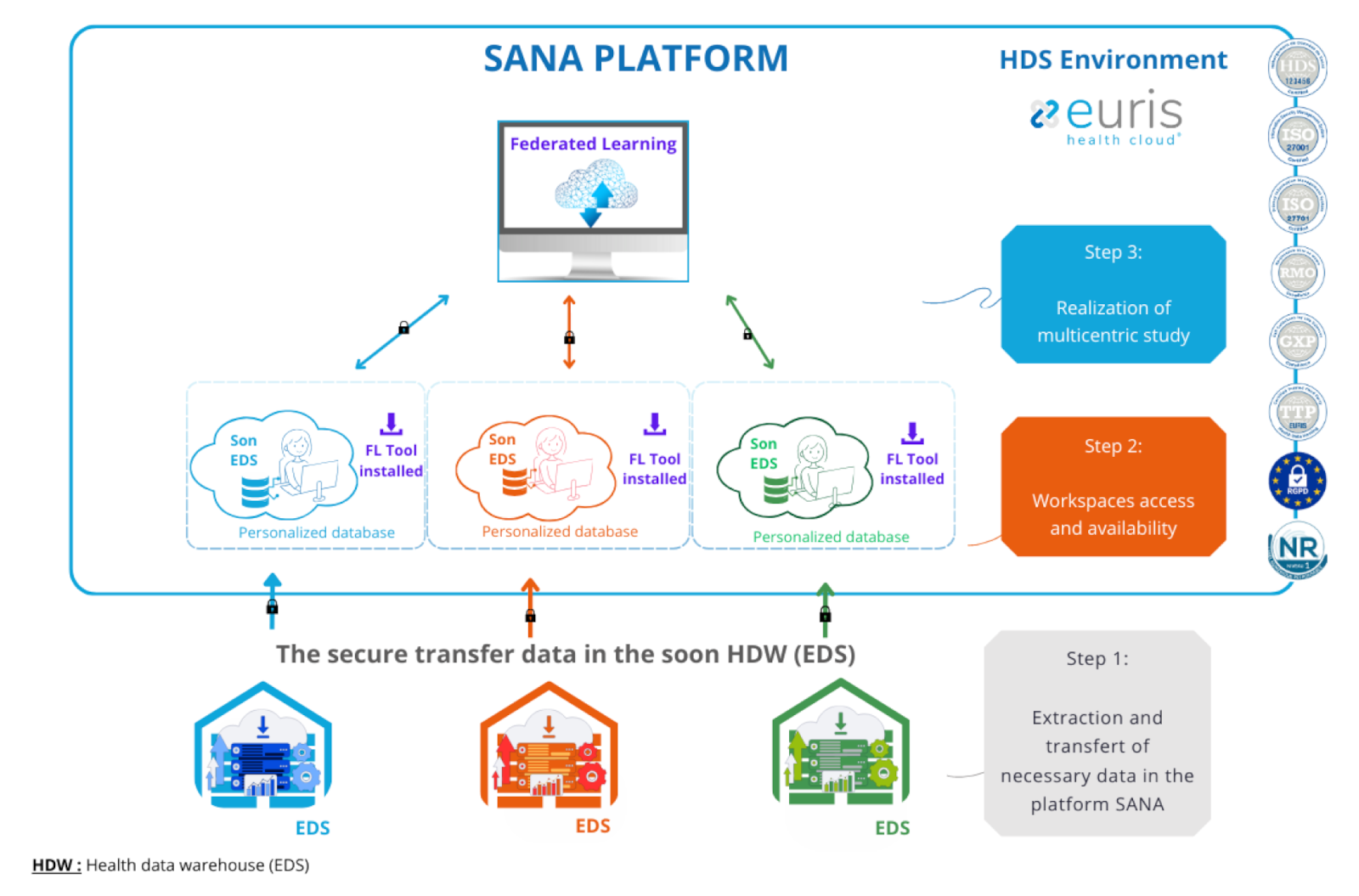
SANA is a compliant workspace and secure storage environment that allows the treatment and analysis of personal health data. Stakeholders in multicentre studies can extract the data they need for their health data warehouse and transfer it to the SANA platform.
The platform architecture was co-developed with project managers and Euris. The platform is divided into private workspaces where data is stored and isolated from each other. Each workspace contains a Federated Learning tool that enables multicentric studies to be carried out.
The purpose of the SANA platform is to provide a tool that promotes the realisation of multicentre studies by collecting data that is specifically and individually enriched by each project leader/data holder in a shared space for data. This space is intended for:





SANA is the first multicentric study platform who allow you to conduct your clinical research without sharing your data
The Federated Learning is a technology allowing a many remote parties to collaboratively trains a AI model, without sharing data. Each party trains a local model using a private dataset.
Only the local model is sent to the central node to improve the quality of the global model, which benefits all parties. This same training is repetead until all parties are satisfied.
This platform proposed by Euris is intended for private and/or public actors, such as health institutions, institutes or research centers, health cooperation groups, public interest groups, manufacturers of health products (medicines medical devices, etc…), cosmetics manufacturers, pharmacy group or companies accompanying health actors who may, after the completion of the prior formalities provided for by the regulations, carry out “turnkey” multicenter studies whether using a shared workplace or a secure workspace. In the context of theses workspaces, data holders will be considered as data controllers.


